MEDICUS engineering ApS
Founded in 2001 as a research and development company
Focuses entirely on development, production and sales of medical devices
MEDICUS engineering’s quality management system complies with ISO 13485
MEDICUS engineering’s product portfolio includes:
A New Technology for Simple, Cost-effective,
In-clinic Screening

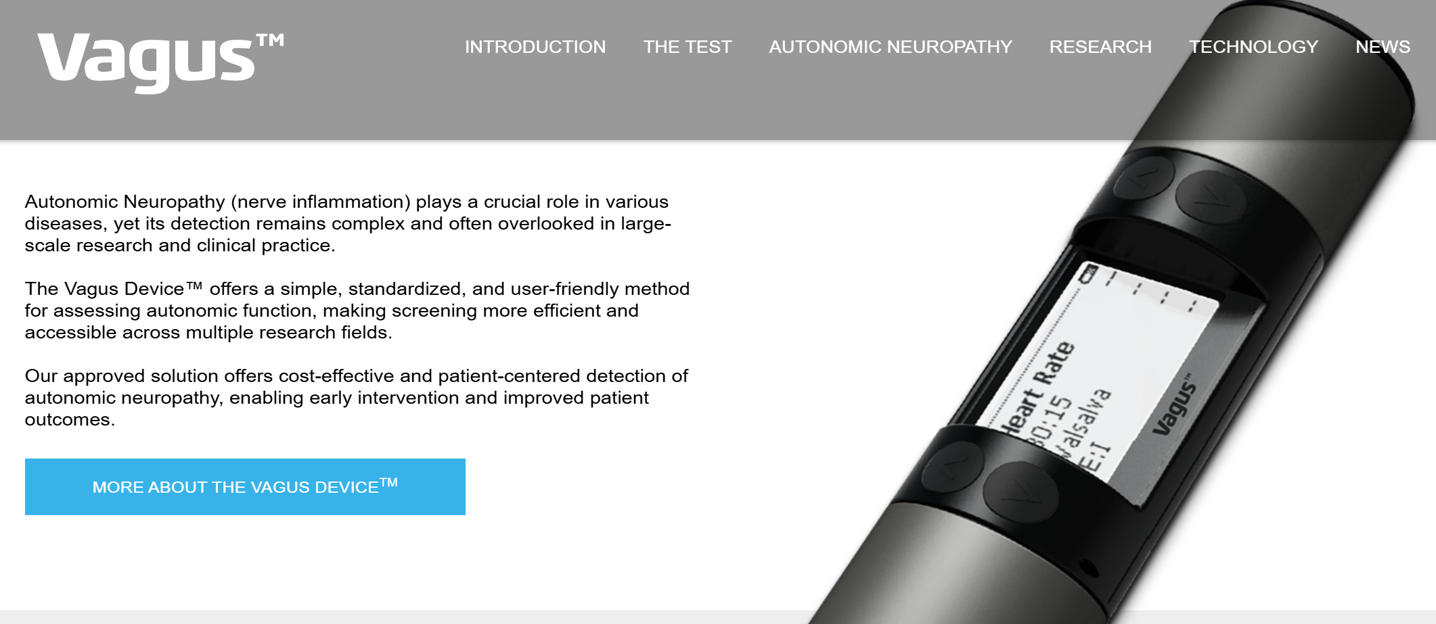
The Vagus Device™ offers a simple, standardized, and user-friendly method for assessing autonomic function, making screening more efficient and accessible across multiple research fields.
Our approved solution offers cost-effective and patient-centered detection of autonomic neuropathy, enabling early intervention and improved patient outcomes.
The Overlooked Impact of Autonomic Neuropathy
Autonomic Neuropathy (AN), a form of nerve inflammation, plays a critical role in various diseases, yet its detection remains complex and often overlooked in large-scale research and clinical practice. Today, 1 in 11 adults—approximately 463 million people—live with diabetes, with 10% of global healthcare expenditure dedicated to managing the disease. Annual costs reach $324 billion in the U.S. and $161 billion in Europe.
The Hidden Burden of AN in Diabetes
After 10 years of living with diabetes, up to 50% of patients develop AN. This condition significantly increases health risks, with patients facing a five-fold higher risk of mortality due to severe complications such as cardiac arrhythmias, silent myocardial ischemia, and sudden death. Additionally, AN is linked to other serious but preventable complications, including hypoglycemia, blood pressure instability, and sleep apnea.
The Urgent Need for Screening
Recognizing its impact, international guidelines recommend annual AN screening for all diabetes patients. While various diagnostic tools exist, current laboratory-based systems are often complex, costly, and inaccessible, limiting widespread adoption and early detection. Addressing these challenges with efficient, patient-friendly solutions is crucial for reducing preventable complications and improving long-term outcomes.
A New Technology for Simple, Cost-effective, In-clinic Screening
Validtated and in use:
With more than 40 peer-reviewed publications and adoption in over eight countries, our technology is trusted by leading pharmaceutical companies to refine and optimize drug development.
Streamline Patient Screenings:
The Vagus Device enables quick, comfortable assessments by in-clinic staff.
Enhance Diagnostic Precision:
The Vagus Device allows for the accurate, non-invasive diagnosis of autonomic neuropathy,aiding in the early and targeted intervention.
Boost Clinic Revenue and Save Time:
The Vagus Device improves clinic efficiency without significant overhead costs. Lowmaintenance and ease-of-use mean seamless integration into existing workflows.
Learn more at: VagusDevice.com
AI-Powered Service for Advanced Prediction
Autonomic Neuropathy (AN) is tied to symptomatic as well as asymptomatic hypoglycemia, which can have a significant impact on a patient's daily life.
PreHypo is an innovative solution for CGM manufacturers looking to improve their devices with AI-driven prediction. By combining advanced machine learning with CGM data and biomarkers such as heart rate, we can revolutionize the diabetes management market and give manufacturers a competitive advantage. PreHypo is clinically validated through extensive studies and millions of data points, proving its accuracy and effectiveness.
Learn more at: PreHypo.com
Quality and Download
MEDICUS quality management system Complies with the requirements of: ISO 13485:2016/NS-EN ISO 13485:2016
-

VagusManual
VagusManualENG-DK v3.0_17 01 2019
Contact
MEDICUS engineering ApS
Palle Juul-Jensens Boulevard 82
8200 Aarhus N
Denmark
mail@medicuseng.com
NEWS